This vaccine is a game changer
Currently two vaccines exist against rotavirus, but they must be kept refrigerated at all times. The most innovative aspect of the new vaccine is that is heat-stable, so does not require refrigeration. This will make it much easier to reach communities in remote areas who have limited access to health services and who are most in need of the vaccine.
The new vaccine is also adapted to the type of rotavirus most commonly found in sub-Saharan countries.
It is also affordable, with a price of under US$2.5, which is much cheaper than the lowest price of Rotavirus vaccines currently available. This should allow it to be rolled out quickly as part of routine immunisation programmes.
The new vaccine, manufactured by Serum Institute of India Pvt Ltd, will also fill the current supply gaps for the existing vaccines.
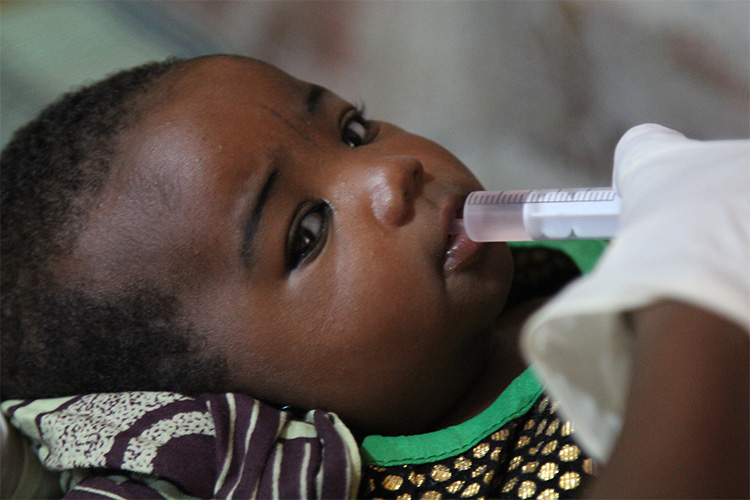
A vaccine trial in Niger
An efficacy trial of the BRV-PV vaccine – carried out by MSF’s research and epidemiology branch, Epicentre, in collaboration with the Nigerien Ministry of Health, the Serum Institute of India Pvt Ltd, the Cincinnati Children’s Hospital and other partners – was recently conducted in Niger’s Maradi region, involving more than 4,000 children under two years old. Results showed that the vaccine has no safety concerns and has been proven to be as effective against severe gastroenteritis as the currently existing vaccines.
The BRV-PV vaccine is currently under review by the World Health Organization (WHO) for prequalification. Once approved, low-income countries will be able to procure the vaccine at an affordable price and roll it out in their countries.
"The success of this trial shows that research and development into vaccines that are specifically adapted for use in low-income countries yields results
The quicker this vaccine is prequalified by the WHO, the sooner it can be used to prevent the deaths of thousands of children in the countries where it is most needed."

